Good Practice on Medicines Expiration Dates
The definition of Medicine Expiry is: The expiry date of a medicine is the point in time when a pharmaceutical product is no longer within an acceptable condition to be considered effective for a patient or has the potential to do harm and the medication reaches the end of its useable ‘shelf life’. This is not to be confused with a “best before date” for perishable foods where they can be eaten after the best before date if they still look palatable.
Depending on the product, the expiry date may be set as a fixed time e.g. after manufacture, after dispensing or even after opening of the manufacturer’s original container. Certain external factors can affect the expiry of a product e.g. contact with water, changes in temperature, exposure to air or light. Generally, solid dose formulations have a longer expiry date than liquid preparations. The shelf life of a product is determined by either how quickly the breakdown of the active drug occurs or due to the risk of contamination from bacteria or other microbes. Not all drugs deteriorate at the same rate and so expiry dates can vary form product to product.
Falsified Medicines Directive (FMD)
The Pharmacy Services Negotiating Committee (PSNC) highlights its governance on FMD. The FMD is a legal framework introduced by the European Commission, to improve the protection of public health within the European Union. The directive applies since 2 January 2013. The European Commission Delegated Regulation, (EU) 2016/161, supplements Directive 2001/83/EC with rules regarding safety features for the packaging of medicinal products for human use. The regulation was adopted in October 2015. Measures to counteract fake medicines include stricter record-keeping of wholesale distributors, tougher inspections of pharmaceutical producers, an EU-wide quality mark to identify online pharmacies and obligatory safety features on packages.
What does all this mean?
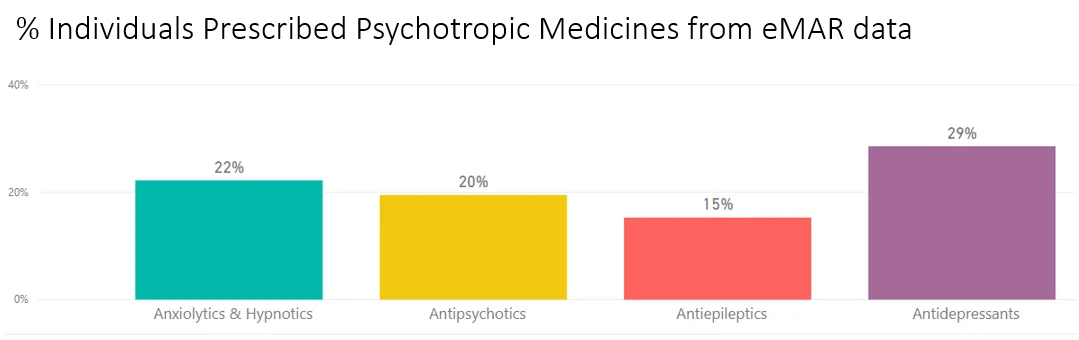
Care and Nursing Homes can unfortunately hold a lot of 'unwanted' stock, also leading to medicines wastage. As part of the FMD, NHS England announced that pharmacists will be sent into care homes to carry out checks on residents, reviewing medicines and stock. Health officials inform that up to 4 in 10 hospital admissions by elderly residents could be avoided if they were given the right care, without over-use of medication. Around 400,000 people live in nursing and residential homes in England - taking an average of seven types of medication daily. This also means that community pharmacies will have to become more vigilant to ensure that they are not issuing 'near-expired' medicines. The FMD also lays down the rules for the manufacture, import, marketing and supply of all medicinal products. The Royal Pharmaceutical Society for Great Britain has also highlighted the importance of FMD governance.
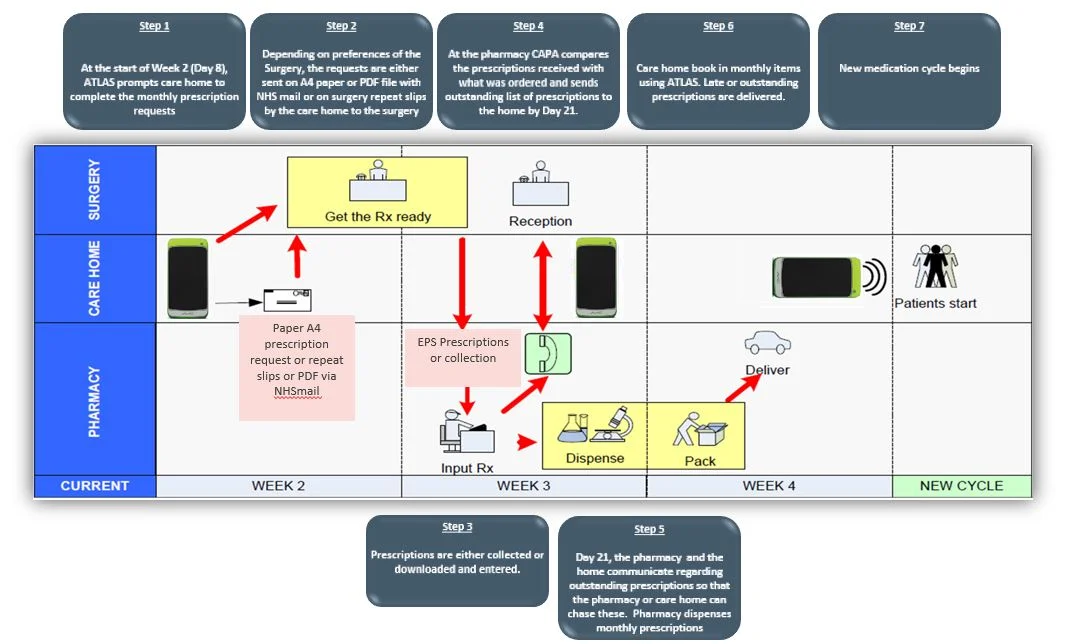
ATLAS eMAR on FMD and Care Homes
Tracking and reviewing stock in care homes helps senior teams’ pinpoint performance breakdowns so they can better understand each part of the process and know which steps need to be optimised to smarter management. FMD is important and plays a role to prevent medicines waste and ensure end-user safety. Technology such as ATLAS eMAR can help smarter medicines management to prevent over-stock.
FMD and Pharmacy Practice
Pharmacists will have to ensure more specific accuracy checks, efficient FMD-specific reporting and authenticating alerts that instantly improve safety. We have taken bar-coding to the next level and has made medicine scanning into the backbone of the entire dispensing process. Only issuing dispensing labels once the manufacturers’ packets have been scanned to ensure that the correct labels are attached to medications.
The Importance of Collaborative Working
All pharmacies that dispense medicines and controlled drugs to patients will have to authenticate medicines before decommissioning them. That means all community pharmacies, hospital pharmacies, dispensing GP practices and non-dispensing GP practices have to become FMD compliant. With this in mind, there is ever-more emphasis importance on strengthening collaborative working between community pharmacy and care homes so patients are not negatively impacted and to ensure best practice on issuing medicines before they expire.